High response rates with single-agent belantamab mafodotin in relapsed systemic AL amyloidosis | Blood Cancer Journal

Extremely High Objective Response Rate of Lenvatinib: Its Clinical Relevance and Changing the Treatment Paradigm in Hepatocellular Carcinoma | Semantic Scholar
![PDF] Objective response rate assessment in oncology: Current situation and future expectations | Semantic Scholar PDF] Objective response rate assessment in oncology: Current situation and future expectations | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/31979901b045184c8053075e4956319c5588d33c/4-Table1-1.png)
PDF] Objective response rate assessment in oncology: Current situation and future expectations | Semantic Scholar

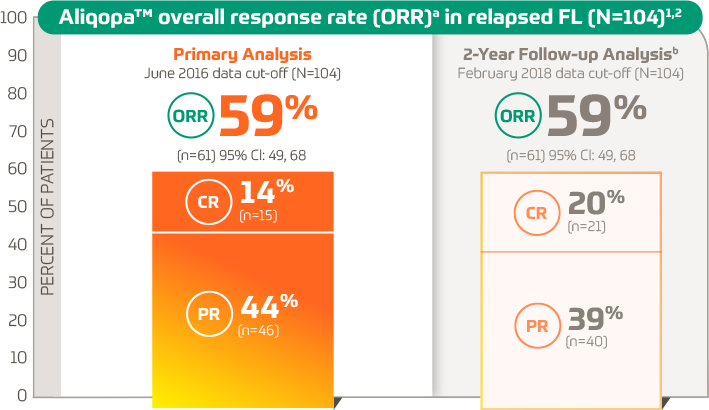
Overall Response Rate in Previously Untreated CLL | CALQUENCE® (acalabrutinib) 100 mg tablets | For HCPs

Overall and complete response rates as potential surrogates for overall survival in relapsed/refractory multiple myeloma | Future Oncology

Overall Response Rate, Progression-Free Survival, and Overall Survival With Targeted and Standard Therapies in Advanced Non–Small-Cell Lung Cancer: US Food and Drug Administration Trial-Level and Patient-Level Analyses | Journal of Clinical Oncology

Objective response rate of placebo in randomized controlled trials of anticancer medicines - eClinicalMedicine
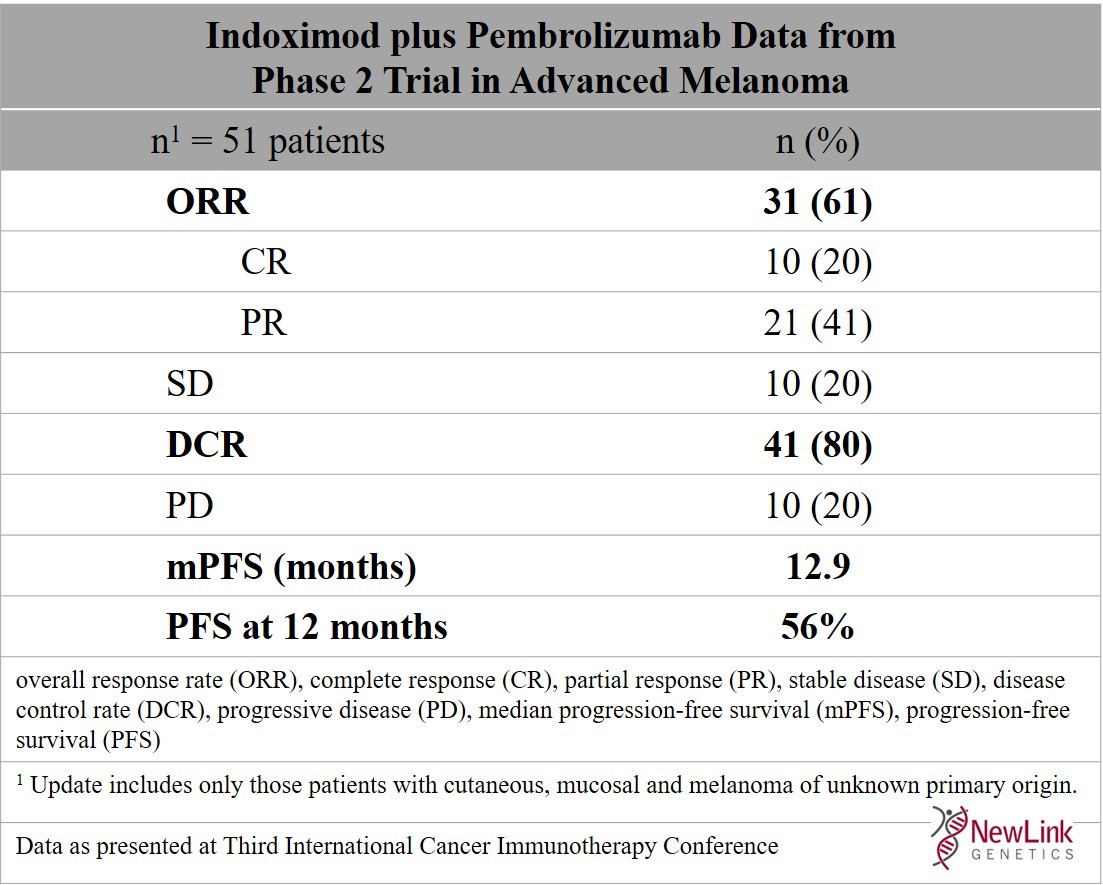
Updated Data for Indoximod Plus KEYTRUDA® (pembrolizumab) Demonstrate Improvement of Response Rate for Patients with Advanced Melanoma | Business Wire

Response rates Overall response rate and depth of response according to... | Download Scientific Diagram